Abstract
The discovery of tyrosine kinase inhibitors (TKIs) has drastically changed the treatment of chronic myeloid leukemia (CML) patients. However, a proportion of patients still show resistance or intolerance to TKI therapy. These patients often carry somatic variants related to myeloid malignancies, indicating that additional mutations other than BCR-ABL1 may lead to TKI treatment failure or disease progression.
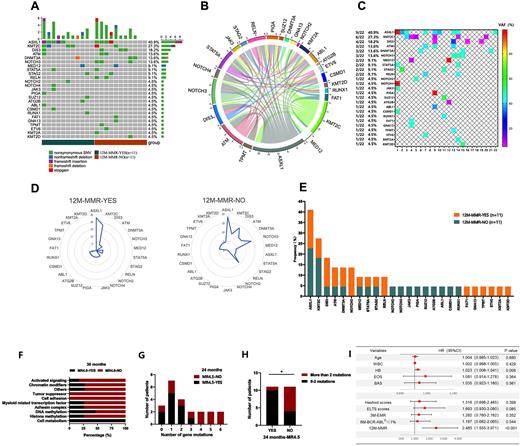
We retrospectively evaluated 151 CML patients receiving TKI therapy and performed next-generation sequencing (NGS) analysis of 22 CML patients to explore the mutation spectrum other than BCR-ABL1 affecting the achievement of molecular responses. We also performed a comprehensive evaluation of clinical characteristics, distinct TKI options and scoring systems in the correlation with the molecular response of TKI. In total, 161 variants were detected by NGS, most of which were nonsynonymous SNVs (Figure A). The coexistence pattern among high-frequency variants was quite intricate(Figure B). Among 25 genes with allele mutation frequency (VAF) ≥5%, the most frequently mutated gene was ASXL1 (40.9%), followed by KMT2C (27.3%), DIS3 (18.2%), ATM (13.6%), DNMT3A (13.2%) and NOTCH3 (13.6%) (Figure C). NOTCH3 and RELN mutations were only carried by subjects failing to achieve a major molecular response (MMR) at 12 months, suggesting CML patients with NOTCH3 and RELN mutations might have poor long-term treatment effects (Figure D, Figure E). The distribution frequency of ASXL1 mutations was higher in the group that did not achieve MR4.0 at 36 months (P=0.023), indicating that ASXL1 mutation was an adverse factor for the achievement of MR4.0. Furthermore, subjects in this group possessed more mutations related to activation signaling pathway genes (P=0.003) and chromatin modification genes (P=0.029) (Figure F). In the group that failed to achieve MR4.5 at 12 months, there were more subjects carrying >2 gene mutations (9.1% vs. 63.6%, P=0.024), implying that it is less likely to achieve MR4.5 with the increase in the number of mutated genes and that the existence of more than two mutations is a poor prognostic factor for achieving deep molecular response (DMR) (Figure G, Figure H). However, no statistical significance was found in the effects of mutations in ASXL1 (P=0.371), KMT2C (P=0.079), DIS3 (P=0.467), ATM (P=0.280), DNMT3A (P=0.479) and NOTCH3 (P=0.479) on PFS. In the analysis of clinical characteristics, multivariate analysis identified hemoglobin concentration (HB) (relative risk, [RR], 1.023; P=0.08) and BCR-ABLIS level at 12 months (RR, 2.485; P<0.001) as independent predictive covariates for MR4.5(Figure I). Subjects who received initial second-generation TKI (2GTKI) were more likely to achieve early molecular response (EMR) at 3 months (P=0.012), BCR-ABLIS ≤1% at 6 months (P=0.012) and MMR at 12 months (P=0.018) than initial first-generation TKI (1GTKI). And there did not show statistical significance in achieving BCR-ABLIS≤1% at 6 months, MMR at 12 months and MR4.5 between original 1GTKI (Group A1, Glevic, Novartis, Switzerland) and generic 1GTKI (Group A2, Genike, Chiatai Tianqing Pharmaceutical Group Co., Ltd.). As for scoring system, ELTS score was sensitive in differentiating whether patients could achieve EMR (P=0.001) and MMR (P=0.004). EUTOS scoring system had a significant difference in achieving MMR at 12 months (P=0.049). However, there was no significant difference in subjects stratified by the Hasford and Sokal scoring systems.
In conclusion, ASXL1 mutation, activation signal pathway genes, chromatin modification genes and the presence of >2 mutations were adverse factors in achieving molecular response with TKI treatment. HB and MMR were independent factors for DMR, and initial 2GTKI therapy was better than 1GTKI in the achievement of molecular response. For the scoring system, we found that the ELTS score was the best in predicting the efficacy of TKI therapy.
Acknowledgement: The work was supported by the Key R&D Program of Zhejiang (No. 2022C03137.), Public Technology Application Research Program of Zhejiang, China (No. LGF21H080003.), the Key Project of Jinhua Science and Technology Plan, China (No. 2020-3-011.), the 2019-2022 Key Medical Discipline (Haematology) Fund of Jinhua, China.
Correspondence to: Dr Jian Huang, The First Affiliated Hospital of Zhejiang University School of Medicine. No. 79 Qingchun Road. Hangzhou, Zhejiang, PR China.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.